21 Technical Sections To Write
to do!
Explain basic Clinical Trial Simulation (Simulation/Estimation * 1000)
Explain key concepts around how we define/assess precision of DR
Explain key concepts around how we define/assess bias of DR
More advanced topics for optimal designs of DR, like D-optimality and V-optimality
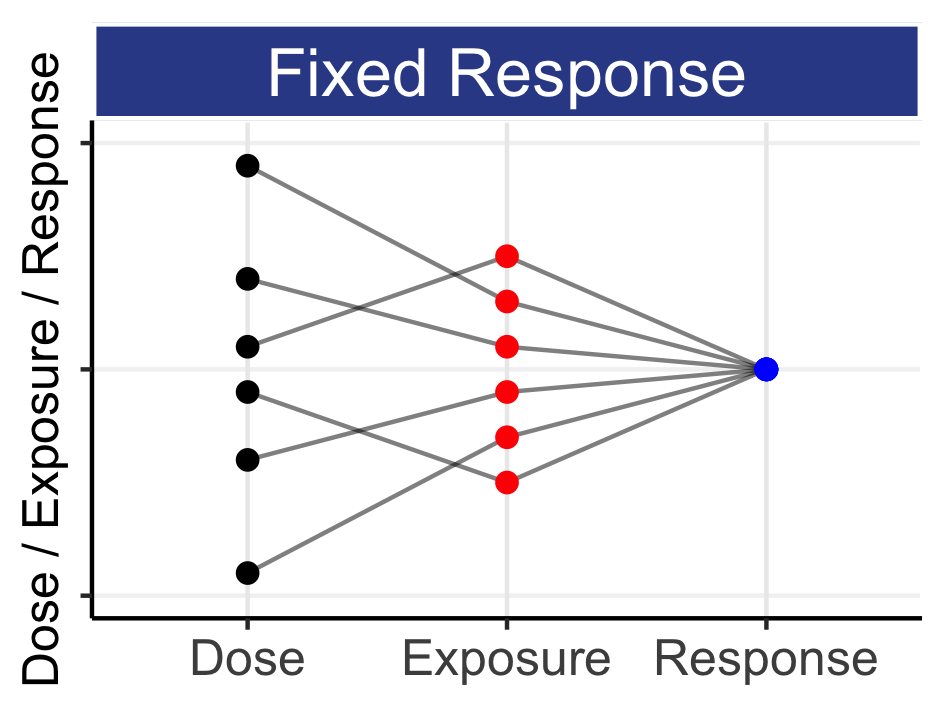
Compare optimal(adaptive) designs of D-E-R versus DR
Introduce Planned Titration trials (a much better name for “forced” titration!)
Explain the value of ER models for prediction (extrapolation to new regimens, pediatrics etc.)
Explain the benefit of dose titration with respect to tolerance
Have sections entitled:
Design and Analysis of Population D-E-R Trials
Design and Analysis of Individual D-E-R Trials
Introduce technical code to fit D-E-R models? e.g. Bayesian analysis using HMC in STAN? Mention diffuse priors (better in Appendix?)
Show case examples where drugs are individualised (to remove the mystery!)