10 Where Does Precision Medicine And Personalised Medicine Fit In?
At the end of this chapter, the reader will understand:
What is meant by the terms precision medicine/personalised medicine.
How precision medicine will help further refine inclusion/exclusion criteria for clinical trials.
That precision medicine is both old and new; a new name for something that has existed for a very long time.
That precision medicine will not be able to determine the right dose for each and every patient.
Our patient level data is ever increasing both prior to, and during, drug treatment. Our goal must be to actually utilise this information to deliver better outcomes for patients.
When discussing Drug Development For Patients, we need to cover two terms that are frequently used in the discussion around individualized treatment regimens; precision medicine and/or personalised medicine. I see these terms used interchangeably, so for the remainder of this chapter I will only refer to precision medicine.
In 2015, Barack Obama famously launched the “Precision Medicine Initiative”. Under the banner “It’s health care tailored to you”, it stated:
“Until now, most medical treatments have been designed for the ‘average patient.’ As a result of this ‘one-size-fits-all’ approach, treatments can be very successful for some patients but not for others. Precision Medicine, on the other hand, is an innovative approach that takes into account individual differences in people’s genes, environments, and lifestyles. It gives medical professionals the resources they need to target the specific treatments of the illnesses we encounter, further develops our scientific and medical research, and keeps our families healthier.”
This is excellent. Clearly we should always strive to use individual patient characteristics to better tailor drug regimens. Similar statements about precision medicine have also been made in the mainstream medical literature [1], such as:
This identification and use of patient-level characteristics to deliver better outcomes for patients is often termed ‘precision medicine’ “
The astute amongst you will recognise that this is nothing new. We have been using individual patient characteristics to tailor individual treatment regimens for a very long time. For example, for over 70 years we have been using a patient’s blood type to ensure transfusions of compatible blood, or using a patient’s body weight to determine the dose of a given drug [2]. Indeed the role of pharmacogenetics is not new. In 2004, Lesko and Woodcock [3] wrote:
“Pharmacogenomics and pharmacogenetics provide methodologies that can lead to DNA-based tests to improve drug selection, identify optimal dosing, maximize drug efficacy or minimize the risk of toxicity.”
Precision medicine has also been defined as a tool for selecting patients for clinical trials who may be expected to benefit most. For example, Cook [4] wrote:
“Precision medicine is an approach to developing drugs that focuses on employing biomarkers to stratify patients in clinical trials with the goal of improving efficacy and/or safety outcomes, ultimately increasing the odds of clinical success and drug approval.”
Again, this is nothing new. We have frequently selected specific patient sub-populations based on the expectation that they will benefit most for the given drug regimen. For example, in the development of statin therapies and new oral anticoagulant drugs to prevent future cardiovascular events, it was standard practice to select only those patients with one or more cardiovascular risk factors. Thus although precision medicine will help further refine inclusion/exclusion criteria for clinical trials, such stratification of patients for clinical trials based on individual patient characteristics is not new.
Perhaps one difference now is the further emergence and availability of more “-omic” data, defined by Wikipedia as:
“…various disciplines in biology whose names end in the suffix -omics, such as genomics, proteomics, metabolomics, metagenomics, phenomics and transcriptomics. Omics aims at the collective characterization and quantification of pools of biological molecules that translate into the structure, function, and dynamics of an organism or organisms. The related suffix -ome is used to address the objects of study of such fields, such as the genome, proteome or metabolome respectively”
In addition, we have an ever-increasing capability to measure and use imaging data, biomarkers and PROs to track both disease progression and disease modification from our pharmacological interventions (e.g. PROs could be recorded in a similar way to fitness trackers like Fitbit).
Thus our patient level data is ever increasing both prior to, and during, drug treatment. Our goal must be to actually utilise this information to deliver better outcomes for patients.
Recall when we put individual patients outcomes first, the 3 steps we seek to understand in drug development are:
Given a patient’s individual characteristics, what is the best initial drug and dosing regimen?
If/when the initial dosing regimen needs to be changed for efficacy and/or safety/tolerability, how best to do this; what is the best science-based dose titration algorithm? That is, based on clinical endpoints, biomarkers, imaging and/or patient reported outcomes (PROs), when should the dose be changed, and by how much.
Under what circumstances should the dosing regimen be halted?
Thus precision medicine will help at step 1) here. Based on a better understanding of the patient, their disease, and the mechanism of action of the drug, we should be able to better select a drug for a given patient. Indeed, in a paper entitled “Drug Dosing Recommendations for All Patients: A Roadmap for Change”, Powell et al. [5] nicely lay out how individual patient characteristics can and should be used to determine an initial dose for patients.
However, it is wholly mistaken to equate any initial dose recommendation (=guess) as the same as the optimal dose for a patient [6].
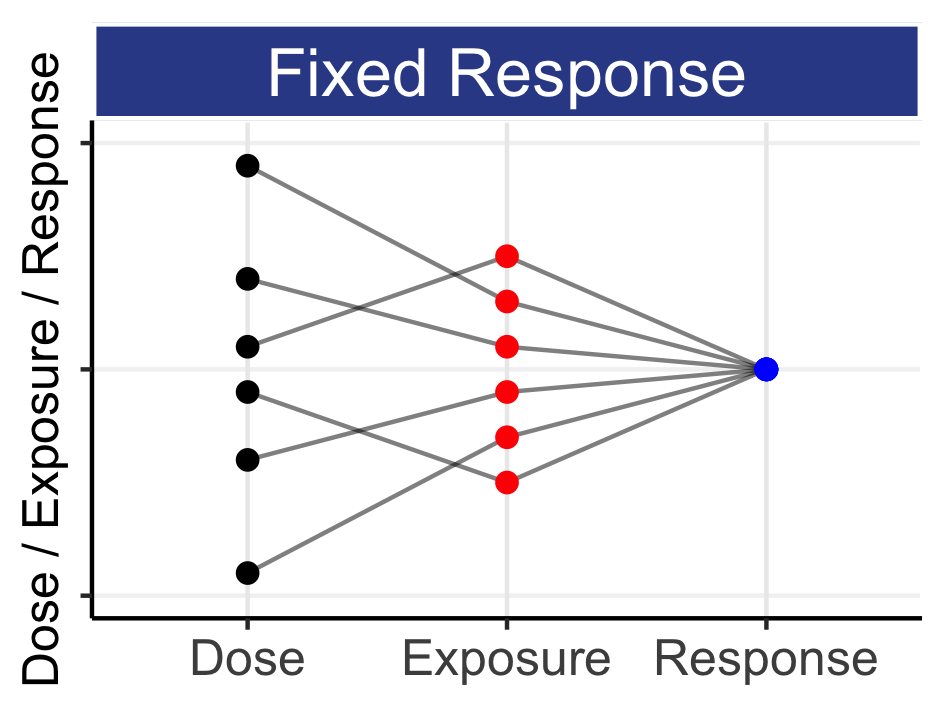
Unfortunately we are nowhere near this level of “predictiveness” for PD endpoints. For anyone who thinks that precision medicine will lead to a revolution in how doses are determined, I would encourage them to study the history of warfarin, and our best efforts at prospectively determining the right dose of warfarin for each patient [7]. Despite a well-understood PD cascade of coagulation, and the use of individual patient characteristics including genetic polymorphisms, the determination of the “optimal” dose for a given patient still requires careful dose titrations using on-treatment PD measures; we simply cannot accurately predict the required dose for a given patient based on their individual patient characteristics alone (as with insulin, anaesthetic agents etc.). Indeed, we have 50+ years of working with much simpler PK data, yet still we have limited capabilities to explain most of the IIV we see across our heterogeneous patients. Rather, we may use these individual patient characteristics to suggest a better initial dose, but must then use dose titration to achieve the desired PD response, since our heterogeneous patients are, alas, heterogeneous.
The mantra
“Right drug, at the right dose, to the right patient, at the right time.”
has been used as one “vision” of precision medicine. Indeed, we would love to be able to be predictive of, say, a week 16 outcome for a patient based solely on their individual patient characteristics and “-omic” data. However I would provocatively suggest that this information combined would struggle to beat the predictiveness of, for example, their week 4 outcome. I particular like this sentence from Kristensen [8]:
“Precision dosing moves beyond the common adjustment of the dose based on body size, demographics factors, renal or hepatic impairment, concomitant medication, etc., and can be guided by observed drug exposure, biomarkers of response or even observed response”.
That is, Personalised Dosing is about using on-treatment PD data to intelligently guide dose modifications, and not just using baseline patient characteristics to “magically” determine the right dose for each patient. In addition, even if we could be much better at predicting objective measures of treatment success for any given dose for a particular patient, predicting the subjective assessments of this patient will remain elusive (we cannot tell patients how they should feel!).
Advances across multiple scientific disciplines have always driven drug development, and always will. Further understanding in pathophysiology and pharmacology will continue to drive the design and evaluation of novel drug molecules. Some of these new treatments will be truly fantastic, but I would argue that this has always been the case. In addition, I am unaware of any precision medicine approach that can tell us the right dose for each patient. Thus some of the current hype around precision medicine ignores the reality of drug development, the real challenges with poor tolerability/safety, and the central role played by dose. Yes, with a greater understanding of the patient, their disease and the use of “targeted therapies”, we will be better placed to select a drug that may have a greater chance of working than other drugs. However most diseases are complex and multifactorial, the patients are complex and heterogeneous, and IIV in PK and PD will remain our enemy.
Finding the best dosing regimen for each and every patient will invariably require Personalised Dosing using on-treatment measures of efficacy and safety/tolerability to guide appropriate dose titrations.
Developing an excellent science-based dose titration algorithm may not grab the headlines, but it will matter to patients if it improves their outcomes, so it should matter to us.
Perhaps the next time you read another article eulogising how precision medicine will revolutionise drug development, look to see if they mention how the dose for individual patients will be determined. If not, hopefully this book will still be useful 😀.