Drug Development for Patients
Personalised Dosing For All
Welcome

Modern drug development is failing patients; it is painfully outdated and desperately needs to change.
This book explains why we must put individual patient outcomes at the centre of drug development and regulatory approval.
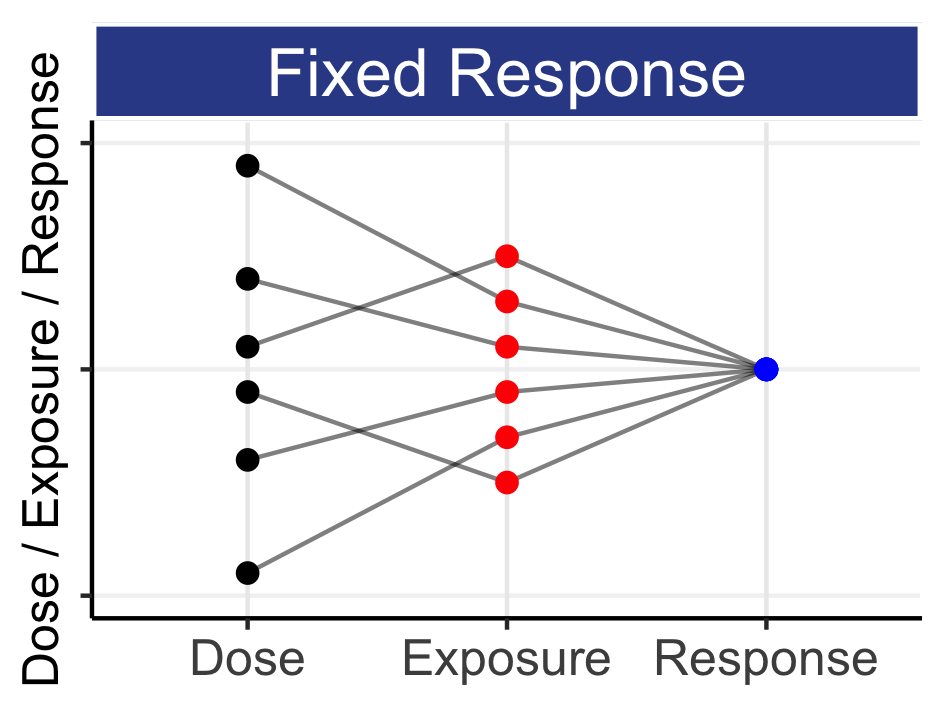
It explains why “one-size-fits-all” dosing is unscientific and incorrect; our (heterogeneous) patients often suffer terribly because we persist with the same “simple” fixed-dose regimen for all. We must refocus drug development to be truly “patient centric” and deliver Personalised Dosing (i.e. dose individualisation). By chapter 4, you will understand that our patients are not “fields”.
The recent draft FDA guidance on dose optimisation in oncology and Project Optimus both aim to improve dosing in oncology; this is excellent, but the continued emphasis on “one-size-fits-all” dosing is incorrect. Thus I have decided to release this (work in progress) book will the goal to both convince and motivate all stakeholders (patients, regulators, pharma, payers) to support a patient focused, Drug Development For Patients paradigm.
These FDA initiatives must state that smart, science-based dose titration algorithms that deliver Personalised Dosing will lead to much better individual patient outcomes than any fixed-dose regimens. If we truly care about obtaining the best individual patient outcomes, we must embrace Personalised Dosing across all therapeutic areas.
After presenting the clear and compelling scientific arguments, I will ask you in the conclusion:
Are you a part of the problem, continuing to accept fixed-dose regimens because you are very familiar with “simple”, even though this leads to worse patient outcomes?
Or are you part of the solution, prepared to embrace Personalised Dosing because you truly care and want the best possible outcomes for our patients?
This is your choice. I hope by the end of this book you will be convinced to choose and actively advocate for the latter; many already do, but we need your voice too!
Hopefully we can all pull together and make it happen.
Happy reading!
Al Maloney 😀